Total Solutions
Evaluation of Medical Equipment/Implant Materials
In-page menu
Outline of Evaluation of Medical Equipment/Implant Materials
-
JFE-TEC can provide a wide range of services, from evaluation tests for medical equipment, beginning with implant materials, to investigation of the causes of damage and defects and mechanical safety testing required for pharmaceutical applications.
- Tests for approval applications (pharmaceutical applications) for medical equipment
- Evaluation tests of orthopedic implants in accordance with ASTM, ISO and JIS standards.
- Various types of evaluation tests for dental implants
-
- The first Japanese laboratory of ISO 17025 accreditation. (mechanical testing)
-
Our Medical Device Testing Center has been accredited as an ISO17025 testing laboratory in the field of evaluation of implant materials.
-
We are the first certified domestic testing laboratory for medical devices (mechanical testing).
-

-
Example of Evaluation of Medical Equipment/Implant Materials
Orthopedic Implants
Material Testing
Spinal Implants
-
Spinal Cages
-
Spinal Rods
Dental Implants
Material Testing
Hip Joints
Material Tests
Knee Joints
-
Material Tests
-
Chemical Analysis
-
- Evaluating the relative extent of oxidation in ultra-high-molecular-weight polyethylene fabricate forms intended for surgical implants
-
Screws
Material Tests
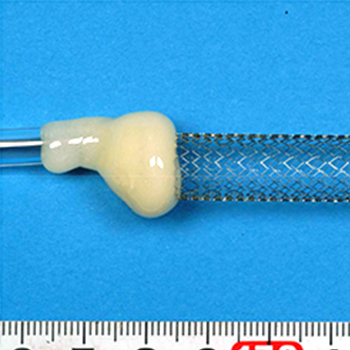
Stents and Catheters
-
Material Tests
-
Corrosion Resistance Evaluation(Corrosion Evaluation)
Wires
Failure Analysis
Material Tests
Chemical Analysis
Corrosion Resistance Evaluation (Corrosion Evaluation)
Surface Observation (Surface Roughness)
MR Compliance Testing
Quick Links to Actual Examples
Example categories
In-page menu
Example details
In-page menu
- * Orthopedic Implants
- - Bending Fatigue Test of Bone Plates
- - Bending Fatigue Tests of Osteosynthesis Devices in Pseudo Physiological Solution
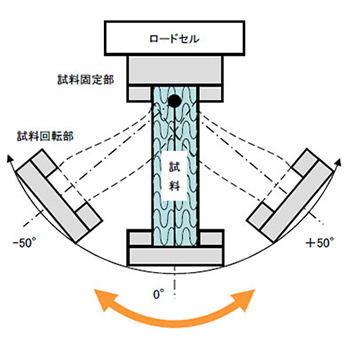
- - Torsional Torque Measurement by Micro Torsion Tester
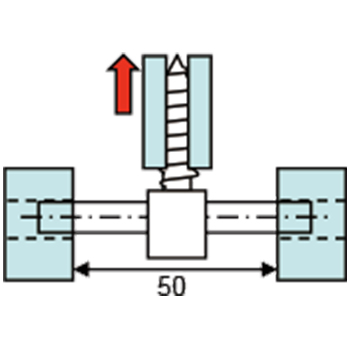
- - Bending Test for Compression Hip Screws (CHS)
- * Spinal Implants
- ** Spinal Cages
- - Static and Fatigue Tests of Vertebra Cages
- - Vertebra Cage Subsidence Test
- - Vertebra Cage Push-Out (Pull-Out) Test
- ** Spinal Rods
- - Measurement of Static Strength and Fatigue Strength of Interconnection Mechanism of Spinal Rods
- * Dental Implants
- - Compression Fatigue Test of Dental Implants
- - Fatigue Test of Dental Implants Using Resin Embedding Material
- - Measurement of Attachment Strength of Attachment for Artificial Teeth (Female)
- - Surface Roughness Measurement of Dental Implant
- * Hip Joints
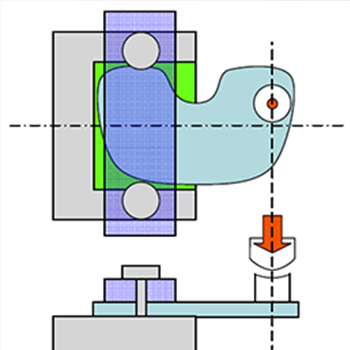
- - Mechanical Testing of Hydroxyapatite Coatings
- * Knee Joints
- - Measurement of Static and Fatigue Strength of Metallic Tibial Trays
- * Screws
- - Precision Torsion Fatigue Test
- - Torsional Torque Measurement by Micro Torsion Tester
- * Stents and Catheters
- ** Material Tests
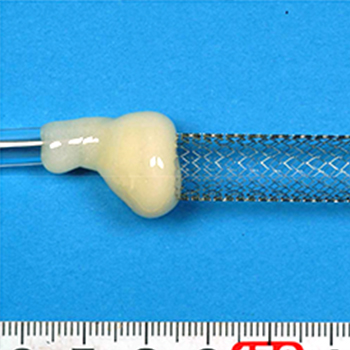
- - Bendability Test of Stentgrafts
- ** Corrosion Resistance Evaluation(Corrosion Evaluation)
- - Evaluation of Corrosion Resistance of Stents (Medical Metallic Components)
- - Corrosion Resistance Testing of Catheters
- * Wires
- - Tensile Test and Fatigue Test of Implant Wires
- * Failure Analysis
- - Failure Analysis/Strength Analysis of Medical Equipment
- * Chemical Analysis
- - Analysis of Metallic Impurities and Residual Catalysts in Medical Products (ICH Q3D Guideline)
- * Corrosion Resistance Evaluation (Corrosion Evaluation)
- - Evaluation of Corrosion Resistance of Metallic Biomaterials
- - Corrosion Resistance Tests of Injection Needles/Syringes
- - Evaluation of Corrosion Resistance of Stents (Medical Metallic Components)
- - Corrosion Resistance Testing of Catheters
- * Surface Observation (Surface Roughness)
- - Surface Roughness Measurement of Dental Implant
- - Observation and Analysis of State of Skin
- * MR Compliance Testing
- - MR Compatibility Evaluation Tests Based on ASTM Standards
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
-----------------------------
Content of Tests for Evaluation of Medical Equipment/Implant Materials (Guide to Commissioned Testing)
JFE-TEC proposes the optimum solution for each client's needs.
-

- Physical Analysis
- State analysis by X-ray photoelectron spectroscopy (XPS) of passivation film, X-ray diffraction
-

- Failure Analysis
- Investigation of causes of failure of orthopedic implants and steel medical products, failure analysis techniques for electronic components
-

- Chemical Analysis
- Surface characteristics test (Ca/P ratio), elution tests of metallic biomaterials (ICP-MS), microanalysis (trace analysis) of organic materials/chemicals
-

- Nondestructive Testing
- High precision stress measurement by infrared camera, radiographic testing, ultrasonic testing, eddy current testing
-

- Finite Element Analysis (FEM)
- Stress analysis of actual behavior by collecting/combining data such as results of vibration test, fatigue test, etc.
-

- Corrosion Resistance Evaluation
- Corrosion resistance testing by anodic polarization test, galvanic corrosion test
-

- Mechanism Safety Tests
- Fatigue tests, tensile tests, bending tests, hardness tests, surface roughness tests
-

- Multi-lingual Translation Service
- Translation of test reports (for example, under ASTM standards, ISO standards, etc.) into English and other languages
Tests of Orthopedic Implants in accordance with JIS Standards
- [ JIS T 0302 ] Testing method for corrosion resistance of metallic biomaterials by anodic polarization measurement
- [ JIS T 0303 ] Testing method for wear resistance of materials for artificial joints by pin-on-disk method
- [ JIS T 0304 ] Testing method for metal release from metallic biomaterials
- [ JIS T 0305 ] Testing method for galvanic corrosion in pseudo physiological solution
- [ JIS T 0306 ] Analysis of state for passive film formed on metallic biomaterials by X-ray photoelectron spectroscopy
- [ JIS T 0309 ] Test method for fatigue properties of metallic biomaterials
- [ JIS T 0310 ] Test method for notch sensitivity and fatigue crack growth properties of metallic biomaterials
- [ JIS T 0311 ] Mechanical testing methods for metallic bone screws
- [ JIS T 0312 ] Testing methods for bending properties of metallic osteosynthesis devices
- [ JIS T 0313 ] Testing methods for compression bending properties of metallic osteosynthesis devices
Reference Standards for Implant Tests
Guidelines for materials to be attached when submitting applications for approval of medical equipment
(Artificial hip joints, artificial knee joints, spinal fixation devices, orthopedic implants, intramedullary nails for internal fixation, dental implants, compression hip plates for internal fixation, cables for internal fixation, pins for internal fixation, screws for internal fixation)
For test reports in English and other languages, just ask JFE-TEC!
JFE-TEC can also prepare test reports conforming to ASTM, ISO and other standards in English as well as Japanese.
We provide translation services for a wide range of languages. Please ask when making an inquiry or request!