Evaluation of Medical Equipment/Implant Materials
Vertebra Cage Push-Out (Pull-Out) Test
In-page menu
Evaluation of the resistance to push-out of a vertebra cage placed between simulated vertebral bodies.
Outline of Vertebra Cage Push-Out Test
This test method is used to measure the resistance to push-out of a vertebra cage, which is an artificial intervertebral spacer used in treatment of spinal disease. Evaluation is performed based on an independent test method referring to the draft standard "Static Push-out Test Method for Intervertebral Body Fusion Devices" prepared by the ASTM (US) technical subcommittee F04.25 (field of technology: spinal devices).
In this test, a vertebra cage is placed between a pair of urethane foam simulated vertebral bodies, and while applying the preload shown in the following table, the load when the vertebra cage is pushed out at a constant speed in the direction perpendicular to the preload is measured.
The push-out load is applied in a direction in which a vertebra cage is easily discharged in the body; the test can also be performed in the pull-out mode, depending on the shape of the vertebra cage.
Conditions of Vertebra Cage Push-Out Test
| Test body | Vertebra cage (intervertebral body fusion device) |
|---|---|
| Test standard | Independent standard prepared referring to ASTM draft standard (ASTM F-04.25.02.02) "Static Push-out Test Method for Intervertebral Body Fusion Devices" |
| Test environment | Constant temperature, constant humidity, in atmosphere (temperature: (23 ± 2)℃, (50 ± 5)%RH) |
| Preload | 100 N (cervical) 300 N (thoracic) 450 N (lumbar) |
| Push-out speed | 6 mm/min |
| Evaluation items | Max. push-out load and displacement at that time |
Vertebra Cage Push-Out Test
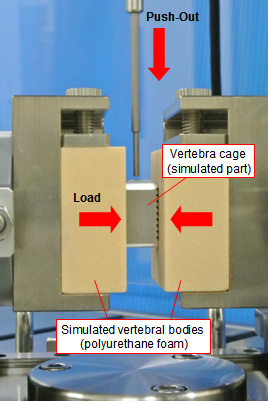
(vertebra cage is the simulated part)
Related Standards and Guidelines for Vertebra Cage
| Title | |
|---|---|
| Test standards | ASTM F2077 "Test Methods For Intervertebral Body Fusion Devices" ASTM F2067 "Standard Test Method for Measuring Load Induced Subsidence of Intervertebral Body Fusion Device Under Static Axial Compression" ASTM F-04.25.02.02 (Draft) "Static Push-out Test Method for Intervertebral Body Fusion Devices" |
| Audit Guideline | FDA Guideline " intervertebral body fusion devices (published June 12, 2007)" |
We can also prepare test reports in English and a number of other languages!
In addition to Japanese, we can also provide test reports in accordance with ASTM, ISO and other standards in English.
We can also provide expert translations in many other languages. Please discuss your language needs with us when making an inquiry or request.


